Platform
Targeted radiotherapeutics consist of three components: a cancer cell-targeting molecule, a chelating agent that binds a radioisotope, and a linker that connects the cell-targeting molecule and the chelated radioisotope agent. The objective is for the cell-targeting molecule to selectively seek out and bind to tumor cells, delivering the radioisotope into the tumors and causing cell death, thus decreasing or limiting the size of the tumor.
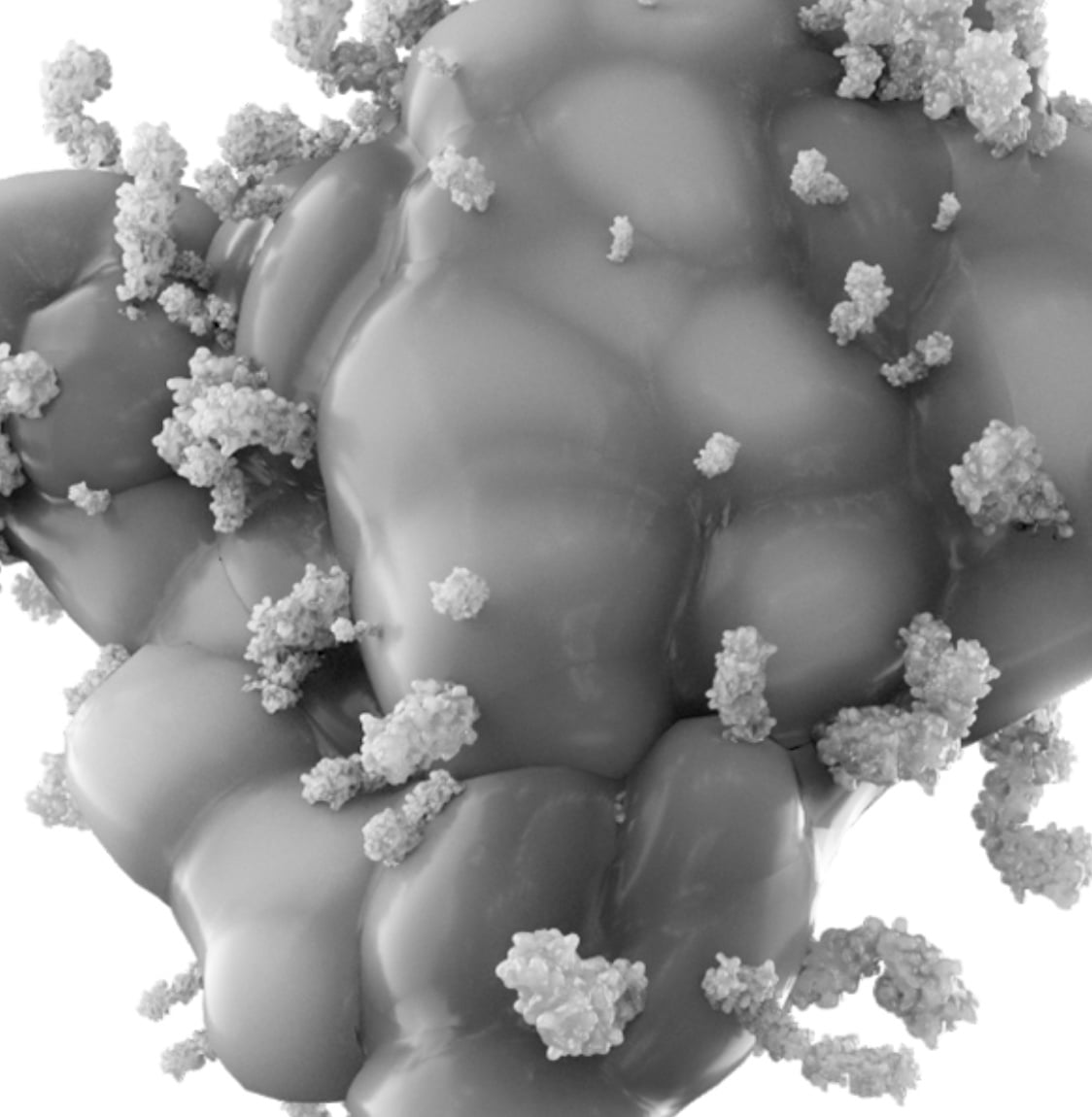
Advanced antibody engineering meets radiotherapeutics
Current approaches leverage either small ligands (e.g., peptides or small molecules) or large proteins (e.g., conventional antibodies) for the cell-targeting component. While small targeting ligands may lead to rapid tumor penetration and excretion, they are often associated with low total tumor accumulation, and high renal uptake of the radioisotope which can lead to kidney toxicity. Large formats, often using existing antibodies not designed specifically for radiopharmaceutical therapy, may avoid renal uptake, but their large size and long half-life in circulation may limit tumor penetration and cause systemic irradiation potentially leading to bone marrow toxicities. In each case, the therapeutic index is not ideal and may limit the ability of these approaches to most effectively treat patients’ cancer.
Abdera’s differentiated approach to radiopharmaceutical drug discovery and development leverages the capabilities of our Radio Optimized Vector Engineering (ROVEr™) platform. We custom-engineer our medicines with tunable pharmacokinetic (PK) properties to achieve an ideal balance of tumor uptake and penetration while avoiding rapid renal excretion, high kidney uptake and mitigating other systemic radiotoxicities such as myelosuppression. Through this platform, we are able to optimize the delivery and therapeutic index of potent radioisotopes capable of emitting powerful alpha or beta particles to selectively destroy tumor cells while sparing healthy cells, providing patients with potentially transformative new cancer treatments.
Our approach offers the ability to design radiotherapeutics against virtually any cancer target expressed on the cell surface. Coupled with a highly potent mechanism of cell killing, the ROVEr platform is uniquely poised to exploit both high- and low-expressing targets to selectively deliver therapeutic levels of radioisotope to cancer cells.
Our Toolbox
Advanced antibody engineering
Our cell targeting vectors are heavy chain only antibodies specifically designed to provide the pharmacology and PK required for radioisotope delivery. These antibodies comprise a high affinity antigen binding domain fused to an engineered human Fc to create an antibody (VHH-Fc) with an optimal size and pharmacokinetic (PK) profile. These are designed to avoid renal filtration, reduce systemic exposure to radioactivity from the blood and enhance uptake and penetration into tumors. Fc engineering serves to fine tune the PK of our antibodies.
Our modular antibody format is designed to allow different antigen binding domains to be switched into the platform to create novel antibodies with predictable PK and pharmacology. This enables the rapid discovery and evaluation of our targeted radiotherapies against any target of interest.
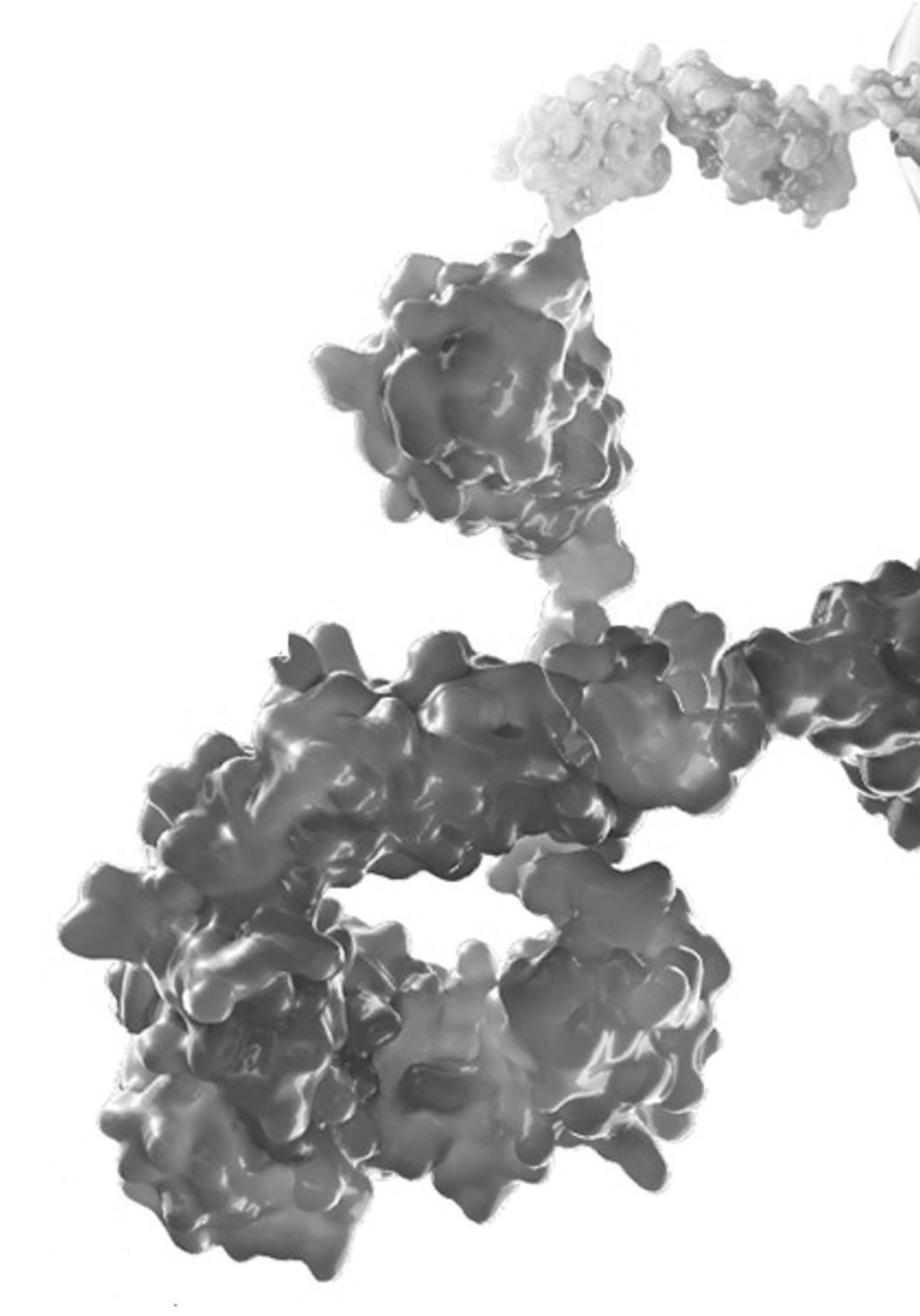
Established linker-chelator library
Our library of linker-chelators are designed to ensure stable attachment of the radioisotope to the antibody and promote clearance from normal tissues. They are compatible with a range of therapeutic radioisotopes including actinium-225 and lutetium-177 as well as imaging isotopes such as indium-111.
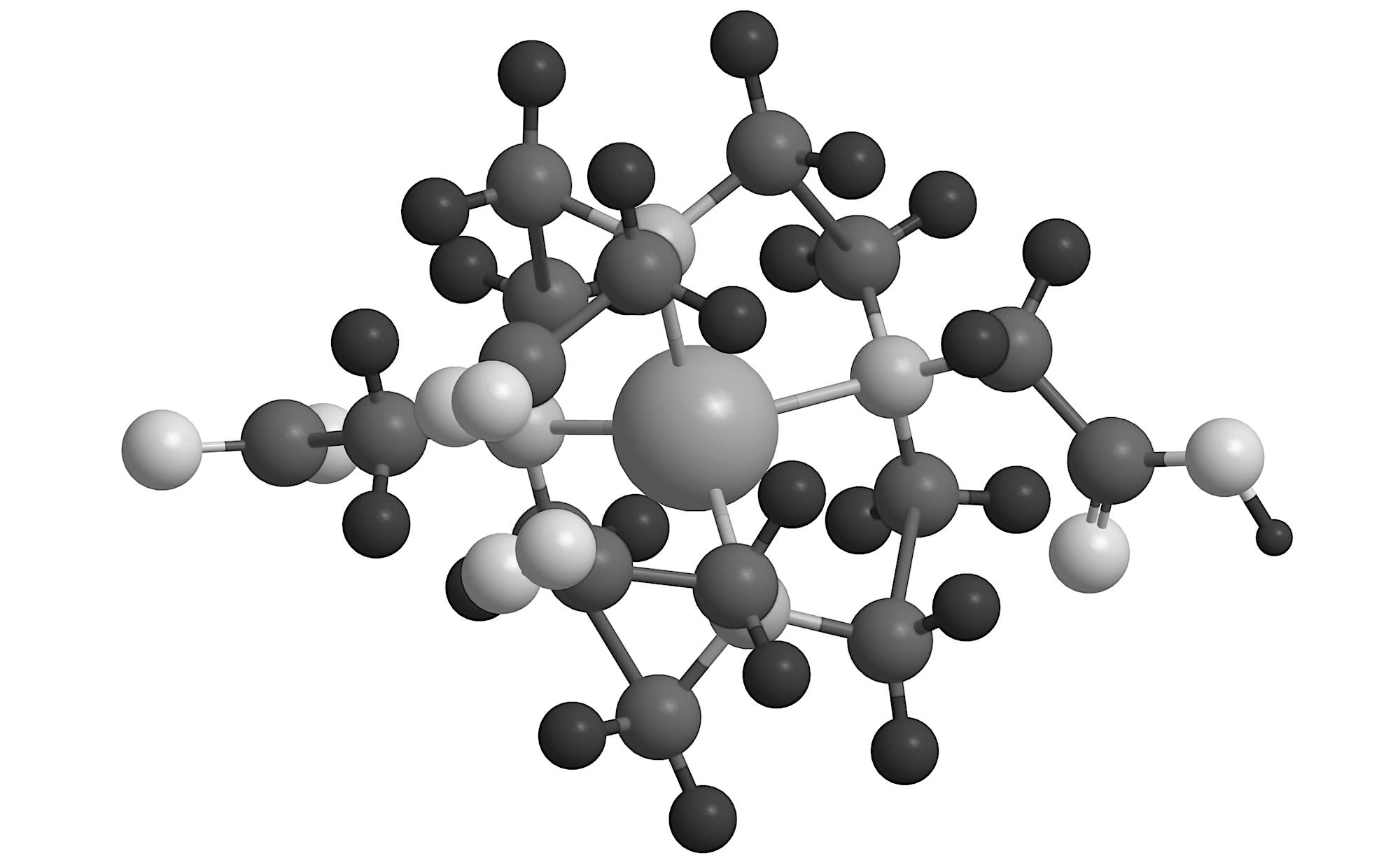
Potent Radioisotopes
Our platform is flexible and radioisotope selection is informed by the desired cancer target and indication. We design medicines that incorporate either alpha or beta-emitting radioisotopes such as actinium-225 and lutetium-177, both of which possess potent tumor-killing abilities.
Image and treat
We are leveraging the inherent ability of our platform to be paired with corresponding imaging isotopes, such as indium-111, to measure target engagement, select the right patients and guide clinical development. Our imaging agents leverage the same antibody-chelator conjugate as the corresponding therapeutic to allow direct data comparison and to streamline development.